Hello again
How to reproduce this structure of NiO if we consider the two atoms of Ni
(up and dn ) as inequivalent.
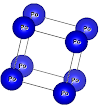
Here is the structure
NiO structure
<http://4.bp.blogspot.com/-EzItbno5RvU/VHi-n_0yT5I/AAAAAAAAAJk/Hyf7Qhg_3ao/s1600/Antiferro-NiO.png>
--
Mr: A.Reggad
Laboratoire de Génie Physique
Université Ibn Khaldoun - Tiaret
Algerie
This problem is quite interesting;
You have Ni in a FCC arrangement, so you have Ni tetrahedra, so how can you
place them in an antiferromangnetic order???
It is frustratng!!! Well this is known as geometric frustration, which is a
very interesting field!!!
On the opposite stance immagine a simple Ni crystal with an antiferromagnetic
order, there is a simple answer; alternating spins in the axis, like the NaCl
crystal: Na represents up, Cl dn;
Now, the picture that you sent the crystal has Ni atoms alternating in spin in
the 111 planes; one plane up, next dn. In each plane the Ni are ordered
ferromagnetic!!!
This is not the correct structure, but it is good for a approximate calculation.
To reproduce this structure you start by defining the structure as shown in
your picture;
You make a crystal with 2 atoms, cubic "F" with Na at 0,0,0 and O at 1/2,0,0.
The conventional structure looks like the one that you sent, but the primitive
one is simpler.
The problem is that when you try to put one Ni plane up and the next dn then
the Ni at 000 is up and the one at 111 is dn, then you have to double the
structure in the 111 direction:
You make the cell with a=b=c=4.17A, alfa=beta=gamma=89.999 (not 90, but close)
and put
Ni 000 0,1/2,1/2 1/2,0,1/2 1/2,1/2,0
O 1/2,0,0 0,1/2,0 0,0,1/2 1/2,1/2,1/2
with sgroup it will convert it to rhombohedral which with supercell you can now
convert it hexagonal and you can expand in the 001 direction (wich in the
original cell was 111)
Here I make the structures step by step
***********************************
Here you have;
aaa.struct-cub-F
with supercell =>
aaa.struct-cub-P
change angles
aaa.struct-89.999
with "initialize calc" and "x sgroup" =>
aaa.struct-rho
with supercell =>
aaa.struct-hex
with supercell =>
aaa.struct-hex-2
here the Ni at
c=0, 1/3 and 2/3 are marked as Ni 1
c=1/6, 3/6 and 5/6 are marked as Ni 2
with "initialize calc" and "x sgroup" =>
aaa.struct-anti
Which is the structure that you want.
**************************
In this "aaa.struct-anti" with F4 you see the hexagonal structure where you see
the Ni-1 planes and Ni-2 planes while with F3 you see the primitive cell.
aaa.struct-cub-F
aaa.struct-cub-P
aaa.struct-89.999
aaa.struct-rho
aaa.struct-hex
aaa.struct-hex-2
aaa.struct-anti








0 Comments